- Incinerator
- Waste Gas Treatment
- Environmental Monitoring
- Product Details
Ozone Denitrification
Technology Introduction
Ozone denitrification is the full name of ozone oxidation combined with absorption denitrification technology. It is an advanced flue gas pollution control technology, which is mainly used to remove nitrogen oxides (NOₓ) from industrial flue gases (such as coal-fired power plants, steel plants, cement plants, waste incineration plants, etc.).
Its core principle is not to directly "remove it", but to use the strong oxidation of ozone (O₃) to oxidize nitric oxide (NO) that is insoluble in water and difficult to remove by conventional methods into high-valence nitrogen oxides that are easily soluble in water, such as nitrogen dioxide (NO₂), nitrous oxide trioxide (N₂O₃) and nitrous oxide (N₂O₅), and then absorb these oxides through subsequent alkali absorption towers (such as scrubbers) to form nitrate (NO₃⁻) and nitrite (NO₂⁻). Thus achieving the purpose of purifying the flue gas.
Working Principle
The whole process can be divided into two core stages:
Stage 1: Ozone Oxidation (Key Chemical Reactions)
The NOₓ in flue gas is mainly composed of 95% NO and 5% NO₂. NO is extremely insoluble in water, while high-valence nitrogen oxides are easily soluble in water.
1. Primary oxidation (formation of NO₂):
NO + O₃ → NO₂ + O₂
NO + O₃ → NO₂ + O₂
This is the primary and main reaction, which rapidly oxidizes insoluble NO to more soluble NO₂.
At this stage, if the ozone dosage (O₃/NO molar ratio) is controlled at about 1.0, the main product is NO₂.
2. Deep oxidation (to produce N₂O₅):
When the ozone dosage (O₃/NO molar ratio > 1.0) is continuously increased, the resulting NO₂ is further oxidized by ozone.
NO₂ + O₃ → NO₃ + O₂
NO₂ + O₃ → NO₃ + O₂
The NO₃ free radicals produced are very active and will react quickly with NO₂ to form N₂O₅.
NO₃ + NO₂ ↔ N₂O₅
NO₃ + NO₂ ↔ N₂O₅
N₂O₅ (nitrous oxide) is highly soluble in water and can be captured very efficiently by subsequent absorption devices. This is the key to high ozone denitrification efficiency.
Summary of oxidation paths:
NO → (O₃) → NO₂ → (O₃) → NO₃ + NO₂ → N₂O₅
NO → (O₃) → NO₂ → (O₃) → NO₃ + NO₂ → N₂O₅
By controlling the ozone dosage and reaction temperature, the type of final oxidation product can be controlled, thereby optimizing the overall removal efficiency.
Stage 2: Absorption of lye
After ozone oxidation, the flue gas enters the subsequent washing absorption system (usually an existing wet dessulfurization tower or a specialized absorption tower).
1. Absorption reaction: High-valence nitrogen oxides (NO₂, N₂O₃, N₂O₅) neutralize water (H₂O) and lye (such as NaOH, Ca(OH)₂) to form stable nitrate and nitrite solutions.
◦ 2NO₂ + 2NaOH → NaNO₃ + NaNO₂ + H₂O
◦ 2NO₂ + 2NaOH → NaNO₃ + NaNO₂ + H₂O
◦ N₂O₅ + H₂O → 2HNO₃ → HNO₃ + NaOH → NaNO₃ + H₂O
◦ N₂O₅ + H₂O → 2HNO₃ → HNO₃ + NaOH → NaNO₃ + H₂O
◦ N₂O₃ + 2NaOH → 2NaNO₂ + H₂O
◦ N₂O₃ + 2NaOH → 2NaNO₂ + H₂O
2. Product treatment: The resulting nitrate and nitrite solutions are discharged as wastewater and enter the water treatment system in the plant for centralized treatment.
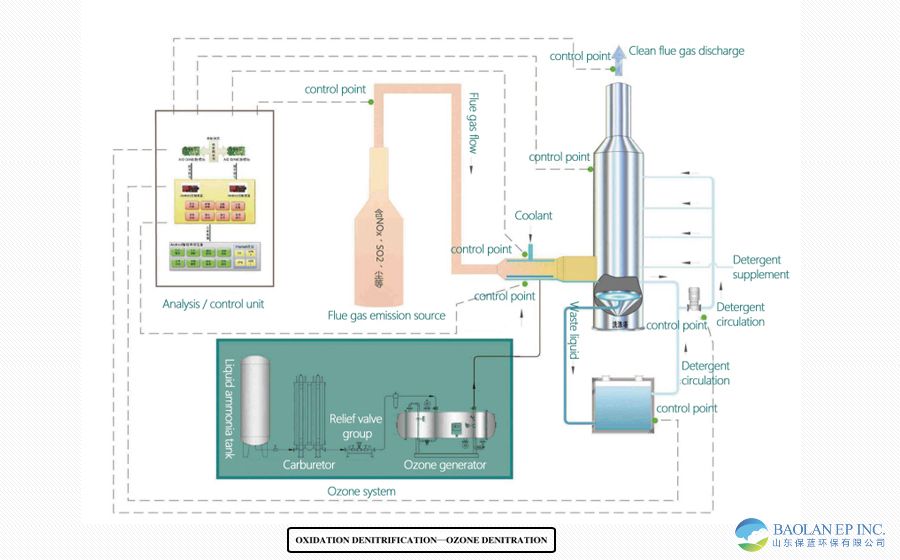
Systematic process flow
A typical ozone denitrification system mainly consists of the following units:
1. Ozone generation system: core equipment. High-purity oxygen or air is used by high-pressure discharge to generate a gas mixture containing a certain concentration of ozone.
2. Ozone Injection System: Sprays ozone evenly into the flue gas through specialized nozzles arranged on the flue.
3. Oxidation reactor: The flue section where ozone and flue gas are fully mixed and oxidized needs to ensure sufficient residence time (usually 0.5-2 seconds).
4. Absorption system: Use the existing wet desulfurization absorption tower or add a special absorption tower to absorb and remove the oxidized products. This is why this technology is often used in conjunction with wet desulfurization (WFGD).
Technical Advantages
1. Efficient denitrification: The denitrification efficiency is high, reaching more than 85%-95%, especially suitable for high concentrations of NOₓ or low-temperature flue gas that are difficult to handle with traditional techniques.
2. No secondary pollution: no problems such as ammonia (NH₃) escape and ammonium salt deposition (compared with SCR/SNCR).
3. Coordinated desulfurization and mercury removal: Ozone can also oxidize sulfur dioxide (SO₂) and elemental mercury (Hg⁰), improving the desulfurization efficiency of the entire system and achieving the coordinated removal of mercury.
4. Sensitive response and flexible control: The system has a fast start-stop and can quickly respond to changes in flue gas load and NOₓ concentration by adjusting ozone production.
5. Simple layout and convenient transformation: The main equipment is outside the flue, and the amount of transformation of the existing process is small, which is especially suitable for the transformation of old factories with limited sites.
Application Areas
Flue gas treatment in coal-fired power plants and gas-fired power plants
Flue gas from sintering machines and pelletizing kilns in the steel industry
Kiln flue gas in glass, ceramics, cement industry
Waste-to-energy plant flue gas
Process exhaust gas in the chemical and refining industries









