- Incinerator
- Waste Gas Treatment
- Environmental Monitoring
- Product Details
Sodium Bicarbonate Desulfurization
Introduction
Dry sodium bicarbonate (NaHCO₃) desulfurization is an efficient flue gas desulfurization process, especially suitable for small and medium-sized boilers, waste incineration, glass kilns and other occasions. The core of the project is the reaction between the active sodium-based substances (Na₂CO₃ and Na₂O) produced by the thermal decomposition of sodium bicarbonate and SO₂ in the flue gas to achieve the removal of sulfur. Here's a detailed description of the process:
Process principle
Thermal decomposition reaction:
Sodium bicarbonate decomposes at 150-250°C into more active sodium carbonate (Na₂CO₃), water, and CO₂:
2NaHCO₃→Na₂CO₃+H₂O+CO₂
2NaHCO₃→Na₂CO₃+H₂O+CO₂
When further heated, Na₂CO₃ is converted to sodium oxide (Na₂O).
Desulfurization reaction:
The Na₂CO₃/Na₂O reacts with SO₂ and O₂ and H₂O in flue gas to form sodium sulfate (Na₂SO₄) and sodium sulfite (Na₂SO₃):
Na₂CO₃+SO₂+½O₂→Na₂SO₄+CO₂
Na₂CO₃+SO₂+½O₂→Na₂SO₄+CO₂
Process flow
Raw material pretreatment:
NaHCO₃ needs to be ground to a fine powder (usually 10-20 μm) to increase the specific surface area to improve the reaction efficiency.
If baking soda (commercial NaHCO₃) is used, it may need to be dried to avoid agglomeration.
Injection system:
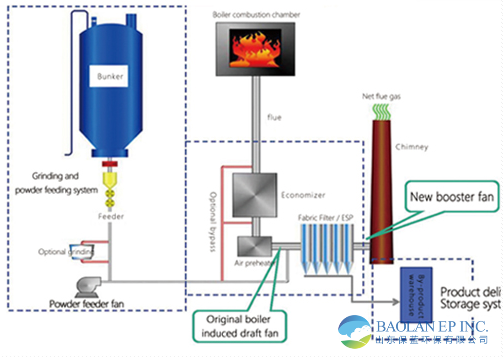
The powder is sprayed into the flue by pneumatic conveying (usually in the flue section in front of the dust collector, temperature range 200-300°C).
The injection point needs to be optimized to ensure adequate mixing with the flue gas.
Reaction Section:
The flue gas is in full contact with the NaHCO₃ powder in the flue, SO₂ is absorbed, and the reaction time is ≥ 1 second.
Reaction efficiency can be improved by flue design, such as increased turbulence.
By-product collection:
The reaction products (Na₂SO₄, Na₂SO₃ and unreacted Na₂CO₃) are captured with flue gas entering the dust collector (such as bag dust collector).
By-products can be used as industrial raw materials (such as glass manufacturing) or safely landfilled.
Key process parameters
Reaction temperature: The optimal range is 140-250°C, too high will lead to sodium salt sintering and inactivation.
Stichiometric ratio: Typically the Na/S molar ratio is 1.5-2.0, ensuring efficient SO₂ removal (up to 95% or more).
Stay time: ≥ 1 second to ensure adequate response.
Flue gas humidity: An appropriate amount of moisture (5-10%) can promote the reaction, but too high can easily lead to equipment corrosion.
Advantage:
Simple equipment, no need for complex slurry systems, low investment.
It is suitable for renovation projects and occupies a small footprint.
Fast start-stop and suitable for intermittent operation conditions.
No wastewater is generated, and the by-products are dry and easy to treat.
Application scenarios
Industrial field: waste-to-energy incineration, glass kilns, ceramic kilns, biomass boilers, etc.
Applicable conditions: projects with medium and low sulfur coal flue gas, small flue gas volume or limited space.
Compare with other dry desulfurization
Comparison with limestone (CaCO₃) dry method:
NaHCO₃ is more reactive and has better desulfurization efficiency, but it is more costly.
Limestone requires higher temperatures (>800°C) and is suitable for circulating fluidized bed boilers.
Comparison with activated carbon adsorption:
Activated carbon can synergistically remove dioxins and heavy metals, but the regeneration is complex. The NaHCO₃ process is simpler.
Dry sodium bicarbonate desulfurization has become the preferred technology in specific scenarios due to its flexibility and efficiency, especially suitable for the treatment of small and medium-sized pollution sources in areas with strict restrictions on wastewater discharge.
Project Case







