- Incinerator
- Waste Gas Treatment
- Environmental Monitoring
- Product Details
Limestone-Gypsum Flue Gas Desulfurization
Technology Introduction
Lime-gypsum method, also known as the wet limestone-gypsum flue gas desulfurization technology, is currently the most widely used and technologically mature sulfur dioxide (SO₂) removal technology in the world. Its core principle is to use alkaline absorbents (lime or limestone) to chemically react with SO₂ in the flue gas, producing stable sulfites and sulfates, thereby achieving the purpose of SO₂ removal.
Working Principle
The entire process mainly includes the following three stages of chemical reactions:
1. Absorption and Ionization of SO₂
After SO₂ in the flue gas enters the absorption tower, it first reacts with water to form sulfurous acid.
SO₂ (gas) + H₂O → H₂SO₃ (sulfurous acid)
Sulfurous acid is a weak acid and partially ionizes in water:
H₂SO₃ ⇌ H⁺ + HSO₃⁻ (bisulfite ion)
HSO₃⁻ ⇌ H⁺ + SO₃²⁻ (sulfite ion)
2. Neutralization Reaction of the Absorbent
The prepared absorbent slurry (mainly composed of CaCO₃ or Ca(OH)₂) is pumped into the absorption tower. The alkaline substances in the slurry react with the H⁺ ions produced by the above ionization, neutralizing the acidic environment and ensuring that SO₂ can be continuously absorbed and dissolved.
If limestone (CaCO₃) is used as the absorbent:
CaCO₃ (solid) + 2H⁺ → Ca²⁺ + CO₂ (gas) + H₂O
If lime (Ca(OH)₂) is used as the absorbent:
Ca(OH)₂ (solid) + 2H⁺ → Ca²⁺ + 2H₂O
The neutralization reaction reduces the acidity of the slurry, ensuring the continuous progress of the absorption reaction.
3. Oxidation and Crystallization
The Ca²⁺ ions produced by the neutralization reaction combine with SO₃²⁻ ions in the solution to form calcium sulfite (CaSO₃). Then, by forcibly blowing air into the slurry tank at the bottom of the absorption tower, the soluble calcium sulfite is oxidized into stable dihydrate gypsum (CaSO₄·2H₂O), i.e., gypsum.
Ca²⁺ + SO₃²⁻ → CaSO₃
Ca²⁺ + SO₃²⁻ → CaSO₃
2CaSO₃ (solid) + O₂ (gas) → 2CaSO₄ (solid)
CaSO₄ + 2H₂O → CaSO₄·2H₂O (gypsum)
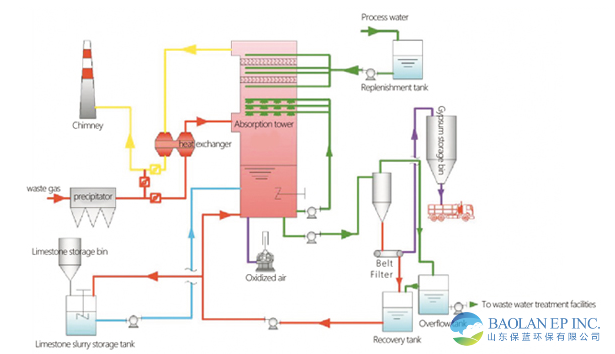
Brief Description of the Process Flow
A complete lime-gypsum desulfurization system typically includes the following key subsystems:
1. Flue Gas System: High-temperature raw flue gas is cooled through a heat exchanger (GGH) and then enters from the middle of the absorption tower.
2. Absorption Tower System (Core):
Spray Layer: The absorbent slurry is circulated by pumps to the spray layer inside the tower, atomizing into countless fine droplets.
Gas-Liquid Contact: The flue gas flows upward and contacts the downward-sprayed slurry droplets countercurrently, undergoing sufficient mass transfer and chemical reaction, absorbing SO₂.
Demister: The cleaned flue gas passes through the demister before leaving the absorption tower to remove carried slurry droplets.
3. Slurry Preparation and Supply System: External limestone powder or quicklime is mixed with process water in the slurry preparation tank to prepare an absorbent slurry of a certain concentration, which is continuously supplied to the absorption tower.
4. Oxidation Air System: Roots blowers or other types of fans blow sufficient air into the slurry pool at the bottom of the absorption tower to fully oxidize calcium sulfite into calcium sulfate.
5. Gypsum Dewatering System: The slurry discharged from the absorption tower (rich in gypsum crystals) is first concentrated by a hydraulic cyclone, then dewatered by a vacuum belt filter, ultimately producing a by-product—commercial gypsum—with a moisture content below 10%, which can be used in industries such as building materials.
Technical Advantages
1. High Desulfurization Efficiency: Usually reaches above 95%, even exceeding 99%, meeting the strictest environmental emission standards.
2. Mature Technology and Reliable Operation: Widely applied, with rich operational experience and high availability.
3. Abundant and Low-Cost Absorbent Resources: Limestone is abundant on Earth and easily accessible.
4. By-products Can Be Utilized as Resources: The produced gypsum has high purity and can be sold as a commodity for manufacturing gypsum boards, cement retarders, etc., generating economic benefits and avoiding solid waste accumulation.
Application Areas
The essence of lime-gypsum desulfurization technology is a chemical absorption-neutralization-oxidation process. It efficiently absorbs acidic SO₂ through alkaline slurry and ultimately converts it into a stable and widely used gypsum by-product. This is a classic and effective technical method for reducing sulfur dioxide emissions from large industrial sources such as coal-fired power plants, steel mills, and chemical plants.










